Oxford, England/oxford989
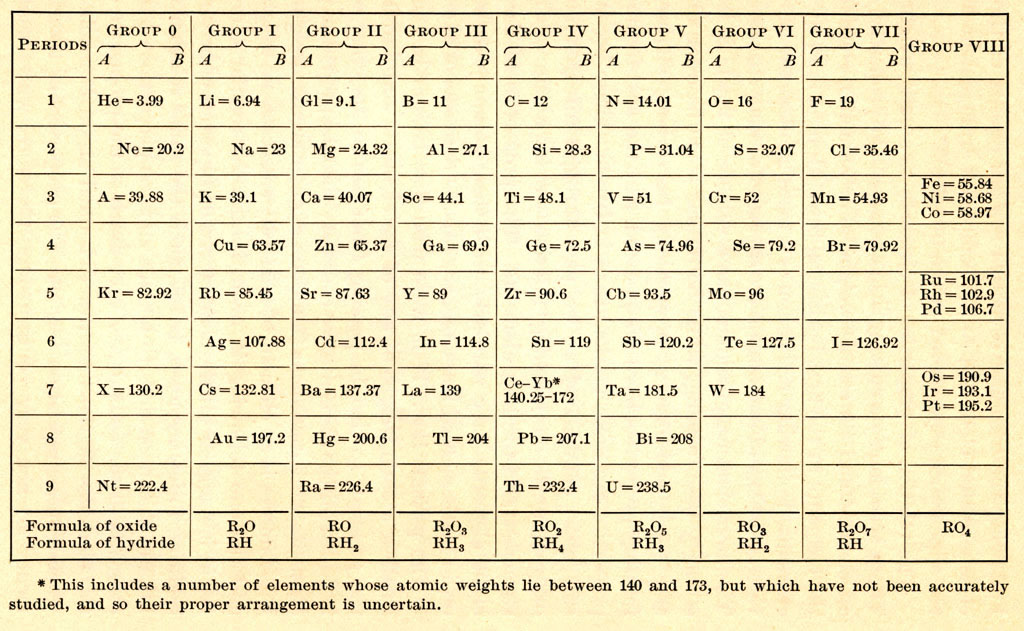
Previous | Home | NextTo help the scholar understand the pre-Moseley Periodic Table, this table is presented, written immediately before Moseley (and published in a 1915 textbook). This table was in arranged as a short-form (doubled-back) arrangement (the commonly accepted format until Bohr explained the Periodic Table in terms of the atom's electronic structure). This 1915 table is accompanied with the explicit statement, "The periodic law states the properties of elements are periodic functions of their atomic weights." Of course Tc, Re, At, Fr, and Pa were missing, since they are yet to be discovered. For some reason, Po and Ac (both discovered in 1898) were omitted, because accurate atomic weight were not yet available. Note that Fe, Ni, and Co were lumped together arranged in order of their atomic weights (instead of their true atomic number order). Also note that the error was made of placing U immediately after Th. Hydrogen (H) was omitted because it "had no place in the table." The Te/I and Ar/K pairs were placed in order of their properties even though it was recognized the atomic weights were reversed for each pair. The rare earths (Ce-Yb) were lumped together with the excuse that they had not been accurately studied yet. (At this time, different chemical symbols were used for some of the elements: Gl = glycinium, the early name for beryllium; Nt = niton, the early name for radon; X and A were early symbols for xenon and argon.)